About the Summit
The 8th Annual DDR Inhibitors Summit - What You Missed
Large pharma like Novartis, AstraZeneca, and Merck are driving the next wave of DDR inhibitor innovation with precision biomarker strategies, cutting-edge ATR and WEE1 inhibitor combinations, and next-gen PARP inhibitors. Meanwhile, emerging biotech leaders, including IDEAYA and ForX Therapeutics, are advancing the field with AI-driven platforms and genetic screening to uncover new targets and develop selective inhibitors.
The 8th Annual DDR Inhibitors Summit in Boston this January brought together these top organizations and many more to collaborate, share insights, and shape the future of DDR therapeutics for cancer treatment.
Key features for 2025:
- Mechanisms of Action Deep Dives: Delve into the intricate biology of DNA damage repair and explore the mechanisms of action for DDR inhibitors, highlighting insights that drive advancements in cancer treatment
- Expert Interactive Sessions: Engage with over 30 industry leaders from top biopharma companies such as AstraZeneca, Novartis, Artios Pharma, and ForX Therpeutics as they share their experiences, challenges, and innovative strategies in assessing clinical biomarker strategies, evaluating combination approaches, and exploring the potential of next-gen PARP inhibitors
- Emerging Therapeutic Targets: Delve into the latest advancements in identifying and understanding DDR pathways, and discuss how novel therapeutic targets like WEE1, POLθ, PARG, CDK12, & FEN1 are showing potential to enhance treatment efficacy and overcome resistance
- Progress Evaluations through Case Studies: Review successful case studies that highlight key milestones in DDR inhibitor trials, such as Debiopharm & Repare Therapeutics’ PKMYT1 & ATR inhibitor MYTHIC trial
- Clinical Trial Design Optimizations: Analyze effective clinical trial designs for DDR inhibitors, focusing on biomarker approaches, patient selection, safety protocols, efficacy measures, and innovative combination strategies
Your Peers Joined To:

Innovative Targets & Combinations:
Assess the potential of combining novel DDR inhibitors with PARP inhibitors to overcome resistance with Artios Pharma, and evaluate radiotherapy and antibody-drug conjugates (ADCs) as DDR combination approaches to localise treatment and reduce toxicity with Zentalis Pharmaceuticals and Wayshine Biopharm.

Predictive Diagnostics:
Hear from Repare Therapeutics on the use of circulating tumor DNA (ctDNA) assessments to guide dose selection and correlate early kinetics with clinical outcomes in the PHASE I MYTHIC trial, and look at Allarity Therapeutics’ multi-gene expression diagnostic designed to accurately predict patient sensitivity to DDR therapies, enhancing the selection process for targeted treatments.

Translational Models:
Investigate the vital integration of in vivo and ex vivo models with Evariste to enhance drug efficacy assessments, and hear from Mayo Clinic on the use of PDX modeling to define target drug concentrations for a novel ATM inhibitor, ultimately optimizing clinical trial outcomes for genetically-defined cancers resistant to standard treatments.

Next-Gen PARP Inhibitors:
Targeted Efficacy: Insights from MD Anderson Cancer Center and Nerviano Medical Sciences into the development of next-gen PARP inhibitors that retain their anti-tumor activity while reducing hematological toxicity and improving delivery across the blood-brain barrier for treating brain metastases.

Future of DDR Therapeutics:
Engage with AstraZeneca and Eikon Therapeutics in a forward-looking dialogue on the evolving landscape of DDR therapeutics, highlighting the role of next-gen inhibitors in clinical validation and the shift towards more patient-centric approaches in treatment strategies.
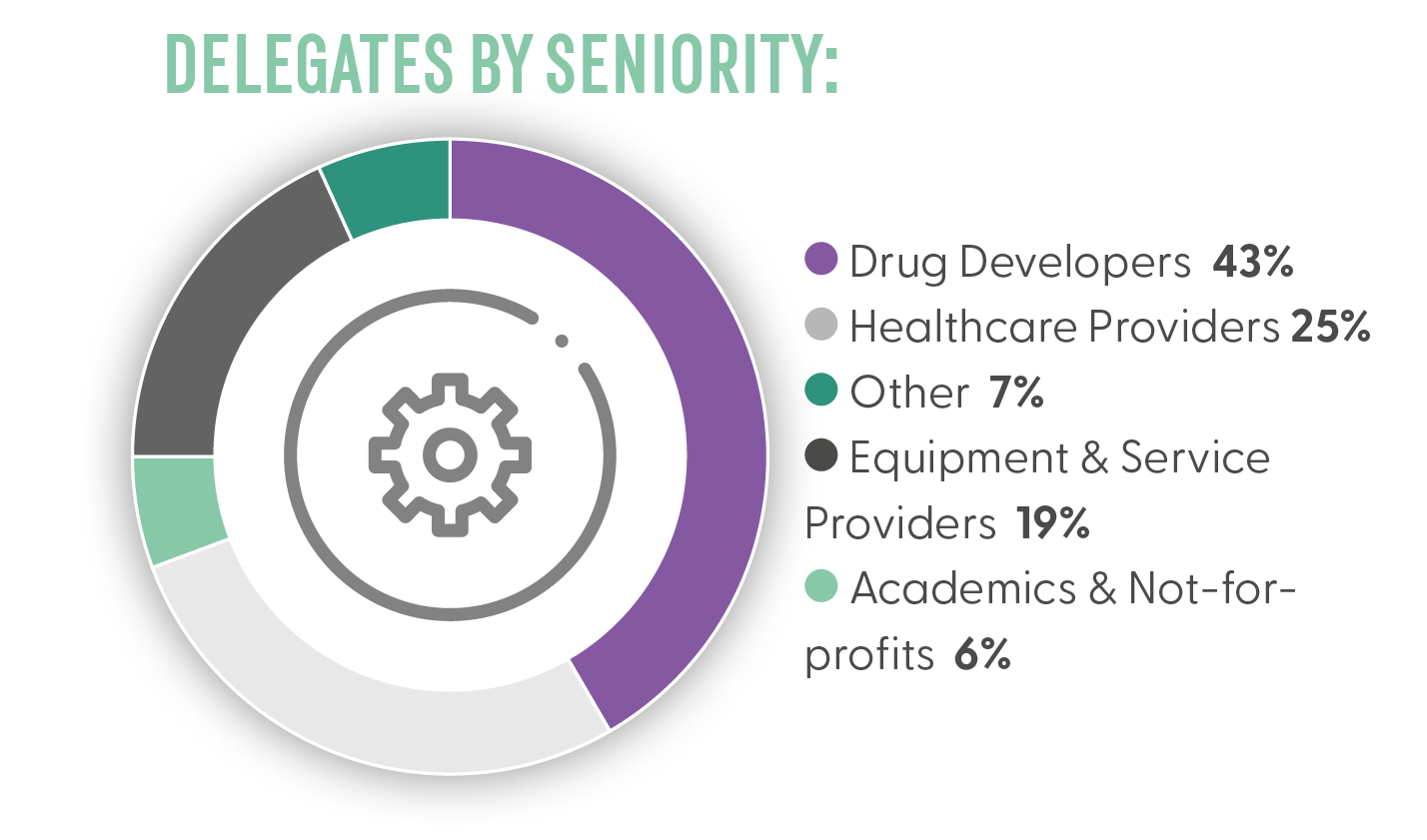
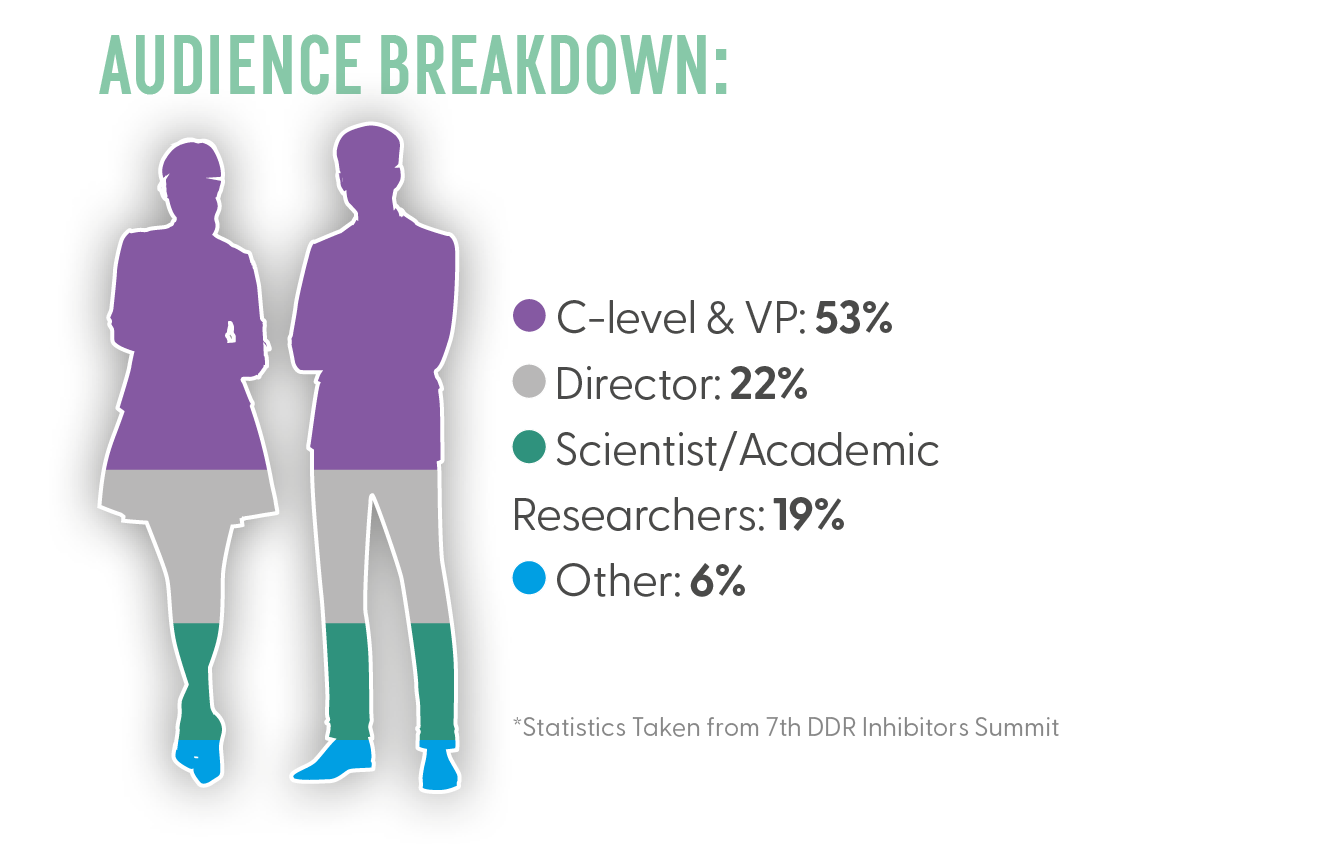
Who You Met?
Hear From Last Year’s Attendees:
“The conference agenda was focused yet covered a variety of different topics and there were many opportunities for group interactions and networking” - Lindsey Rodrigues, Associate Director, Ipsen Pharma


“The most important thing to me was being able to meet people working at essentially all areas of the drug development pipeline. I learned a lot and substantially expanded my network.” - Grant Frost, Cofounder & Chief Scientist, Riptide Therapeutics
“Great and inspirational discussions and ample opportunities to network. Much appreciated.” - Jiyun Chen, Vice President, Translational Science, 1cBio


